Rigorous Science
Need for Dietary Policy Based on Rigorous Science
The Dietary Guidelines for Americans (DGA) have a profound influence on U.S. eating habits. In addition to informing the advice recommended by doctors, nutritionists, and dietitians, the guidelines also drive government nutrition assistance programs, including the National School Lunch Program (NSLP), Supplemental Nutrition Assistance Program education programming (SNAP-ed), Special Supplemental Nutrition Program for Women, Infants and Children (WIC), and nutrition support for seniors. These programs impact one in four Americans every year and are USDA’s single-biggest annual budget item.
With millions of Americans and our most vulnerable citizens relying on the dietary guidelines, it’s imperative that we get them right by basing them on the best-available, most rigorous scientific evidence.
Before the next iteration of the dietary guidelines in 2020, the National Academies of Science, Engineering, and Medicine (NASEM) advised the U.S. Departments of Agriculture (USDA) and Health and Human Services (HHS) to upgrade their scientific reviews to use one of the rigorous, state-of-the-art methodologies.
Unfortunately, the USDA office in charge elected to use its own methodology which is far less rigorous, lacks prioritization of evidence, and cannot guarantee reliable, trustworthy dietary guidelines.
Defining a Rigorous Review Process
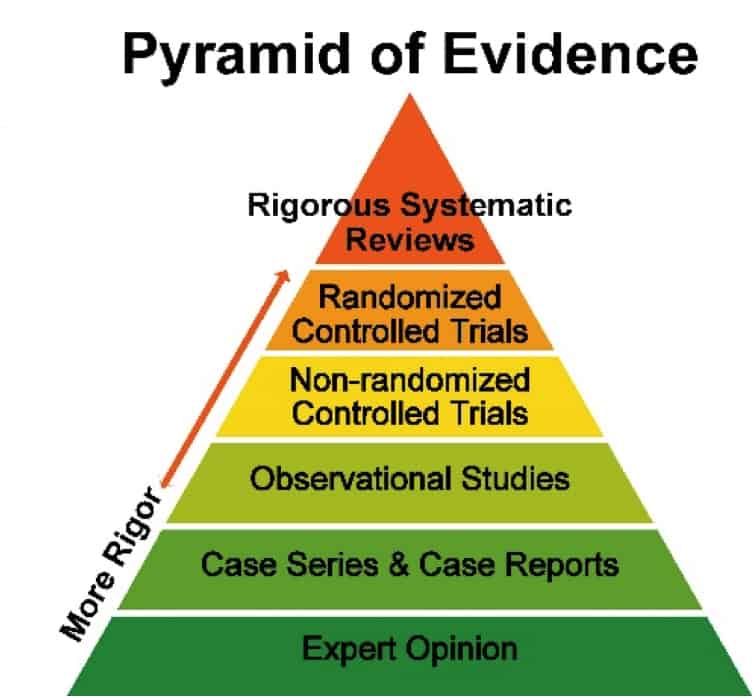
Not all science is created equal, and a rigorous review of the science is necessary to make certain that the foundation of our federal nutrition policy is built upon the most trustworthy and reliable evidence.
In order for a scientific review process to be considered “rigorous,” it requires a formal evaluation of the strengths and weaknesses of studies being considered to ensure proper prioritization of more rigorous, cause-and-effect data over weaker, association-only evidence. This initial prioritization, along with steps to analyze bias, generalizability, and other aspects of the evidence, ensure that resulting recommendations are based on sound, accurate, and reliable evidence. These formal evaluations, known as “state-of-the-art systematic reviews,” are considered the “gold standard” of evidence review and are used to guide both clinical and nutrition guidelines. These reviews provide a comprehensive appraisal of the evidence by minimizing bias and prioritizing strong data.
Examples of leading state-of-the-art systematic review methodologies include:
- GRADE (Grading of Recommendations Assessment, Development and Evaluation)
- Cochrane
- AHRQ (Agency for Healthcare Research and Quality), developed by US-HHS